Treating Combat Related Ptsd and Combat Operational Stress Reaction a Clinicians Review
one Introduction
Mail-traumatic stress disorder (PTSD) is a kind of stress disorder with severe clinical symptoms, poor prognosis, and possible encephalon damage in traumatic and stress-related disorders (Mithoefer et al., 2018). PTSD refers to individuals who have faced unusually strong mental stress, such as natural disasters, traffic accidents, the sudden loss of relatives, and other accidents, which result in the emergence of stress-related disorder. The lifetime prevalence of PTSD in adults is approximately 8% (Kessler et al., 2012; Nichter et al., 2021), between x and 20% of trauma survivors in some cases develop chronic PTSD. This brings considerable distress to society, families, and individuals, and causes huge economic and social burdens, and even increases the chance of suicide (Kessler et al., 1995; Kessler, 2000; Frayne et al., 2004; Krysinska and Lester, 2010; Wittchen et al., 2011).
PTSD is a mutual clinical mental disease, particularly for some high-hazard groups. In Iran, which has survived 8 years of war, most 40% of veterans with PTSD take gainsay-related PTSD associated with more than severe symptoms and farthermost symptom conditions that are harder to treat PTSD (Mithoefer et al., 2018). Current research (Mohamed and Rosenheck, 2008) indicates that medication is a mutual choice for the treatment of combat-related PTSD. Although sertraline and paroxetine are considered of import and common medications for PTSD, their bodily efficacy of selective serotonin reuptake inhibitors (SSRIs) remains controversial (Bajor et al., 2011). In addition, current drug regimens for PTSD do not have satisfactory clinical outcomes, with an approximately threescore% response rate amidst PTSD patients treated with SSRIs (Suttajit et al., 2014). Related studies (Bisson et al., 2019) have focused on the efficacy of SSRI on combat-related PTSD, investigating the feet and depression associated with PTSD. However, the iii core symptoms, including abstention, re-experience, and hyper-arousal, based on the DMS-3 accept not been researched to date (Bisson et al., 2019). Other studies have found that ketamine and other drugs can affect the pathway of specific signals in suicidal tendency handling, which achieves the upshot of rapid feelings of anti-suicide and anti-low (De Berardis et al., 2018). Still, as noesis of neuroscience and the pathophysiology of human behavior go along to advance, suicidal behavior remains a challenge. Although the possible pathophysiological factors that may explicate the circuitous link between PTSD and suicide have been explored in studies, there is little research on the underlying office of neurobiological factors (e.k., genetics, exogenous and endogenous stressors, epigenetic, the hypothalamic-pituitary-adrenal stress-response organisation, the involvement of the monoaminergic neurotransmitter systems, peculiarly the serotonergic ones, the lipid profile), neuro-immunological biomarkers, brain-derived neurotrophic factors, and other neuromodulators (Orsolini et al., 2020). Based on the fact that treating PTSD with conventional drugs tin involve major challenges, including excessive agin furnishings, express efficacy, and lower patient compliance, this report comprehensively evaluates the efficacy, acceptability, and safety of active drugs for PTSD, and explores the influence of relevant confounding factors on PTSD to ensure the reliability and extrapolation of evidence, and provide a reference for clinicians when making handling decisions.
two Methods
2.1 Search Strategy
The Ovid MEDLINE, Ovid EMBASE, Cochrane Library, Scopus, ScienceDirect, and Web of Science databases were searched for randomized controlled trials (RCTs), which had investigated active pharmaceutical assistants in gainsay-related PTSD until Apr 21, 2021. The detailed search strategy is displayed in Supplementary Method S1.
ii.2 Inclusion Criteria and Exclusion Criteria
The included studies met the following criteria: 1) Adult (≥xviii years old) with all combat-related PTSD co-ordinate to diagnostic criteria, including DSM-III, DSM-Three-R, DSM-IV, DSM-IV-TR, DSM-V or ICD-x; 2) Interventions: any dosages and treatment duration of agile pharmaceutical administration, including amitriptyline, aripiprazole, dexamethasone, fluoxetine, guanfacine, imipramine, olanzapine, phenelzine, prazosin, pregabalin, quetiapine, riluzole, risperidone, rivastigmin, topiramate, vilazodone, and other agile drug agents; 3) Comparisons: Placebo; 4) Outcomes: effectiveness (changes in total symptoms of combat-related PTSD, depression, anxiety, re-experiencing, avoidance and hyper-arousal), acceptability [all-cause discontinuation and discontinuation due to adverse events (AEs)], and AEs (somnolence, sedation, dizziness, paresthesia, anxiety and blurred vision, generalized anxiety disorder and slumber disturbance). The change in total symptoms of combat-related PTSD according to the clinician rating calibration was divers as primary outcome, and the others were divers as secondary outcomes. 5) Written report blueprint of included studies: RCTs.
Studies with 1 of the post-obit criteria were excluded: ane) subjects diagnosed with schizophrenia, schizophrenia disorder, bipolar disorder, exhibiting clinically meaning suicidal, homicidal ideation or other dangers; 2) a combination of treatment options other than drug intervention; 3) duplicate data or data could not be retrieved.
2.iii Data Extraction and Quality Assessment
Basic information was extracted, including written report, year, sample, age, gender, battlefield, race, diagnostic criteria, baseline score, dosages of drugs, elapsing of treatment, and interesting outcomes. We used the change values from the baseline as much as possible in all the continuous outcomes. If alter values from the baseline were not mentioned, we used a comparing of final measurements co-ordinate to the Cochrane handbook (Julian and Emerge, 2011), which is a randomized trial estimating the same baseline value in theory.
The quality assessment was employed by the risk of bias based on the Cochrane Handbook for Systematic Reviews of Interventions (Julian and Sally, 2011).
2.4 Statistical Assay
Take a chance ratios (RR) or standardized mean divergence (SMD) with 95% were employed for dichotomous data and continuous data. The heterogeneity of the meta-analyses was measured by calculating the I2 (Higgins et al., 2003). If I2 ≥ 40%, the random furnishings models were employed; otherwise, the stock-still model was adopted. Subgroup analyses were employed for investigating the clinical benefits of overall active comparators in patients of historic period (<60 years and ≥threescore years), race (white and other), gender (male person and female), different battleground, duration of handling, and severity of trauma, based on CAPS scores (60–79 and ≥eighty) based on changes in total symptoms of combat-related PTSD, depression, and anxiety. Funnel plots were used to detect publication bias (Moher et al., 2009). All statistical analyses were employed for the software of RevMan 5.4.1. The Preferred Reporting Items of Systematic Review and Meta-Analyses (PRISMA) past systematic review and meta-analysis (Moher et al., 2009) were employed for the systematic evaluation and meta-analysis.
3 Results
iii.one Report Selection
As shown in the study selection in Figure 1, 8,967 studies were searched from databases, in that location were half-dozen,758 studies left after deleting indistinguishable studies, among which this study used titles and abstracts to filter. We excluded half-dozen,671 studies due to them having unrelated themes. A full-text review was done for the remaining 87 studies and we identified 21 articles (Davidson et al., 1990; Kosten et al., 1991; Hertzberg et al., 2000; Stein et al., 2002; Hamner et al., 2003; Monnelly et al., 2003; Akuchekian and Amanat, 2004; Bartzokis et al., 2005; Neylan et al., 2006; Lindley et al., 2007; Raskind et al., 2007; Davis et al., 2008; Baniasadi et al., 2014; Naylor et al., 2015; Petrakis et al., 2016; Villarreal et al., 2016; Ramaswamy et al., 2017; Rezaei Ardani et al., 2017; Surís et al., 2017; Raskind et al., 2018; Spangler et al., 2020) with 22 RCTs that run across the inclusion criteria for this meta-analysis.
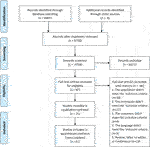
Effigy ane. Literature screening.
3.two Report Characteristic and Quality Assessment
In the meta-analysis, 22 RCTs with 1,221 patients provided an evaluation of the clinical benefit of drugs in combat-related PTSD. Nonetheless, there were 12 RCTs in an all-male population (Kosten et al., 1991; Hertzberg et al., 2000; Stein et al., 2002; Monnelly et al., 2003; Bartzokis et al., 2005; Lindley et al., 2007; Baniasadi et al., 2014; Rezaei Ardani et al., 2017; Surís et al., 2017), and the residual were in a mixed population, lacking RCT of drug therapy in female patients. The bulk of battlefields were Transitional islamic state of afghanistan or Iraq (Spangler et al., 2020), Iran or Republic of iraq (Akuchekian and Amanat, 2004; Baniasadi et al., 2014; Rezaei Ardani et al., 2017), Vietnam (Kosten et al., 1991; Hertzberg et al., 2000; Hamner et al., 2003; Lindley et al., 2007), and unreported or different combat locations (Davidson et al., 1990; Stein et al., 2002; Bartzokis et al., 2005; Neylan et al., 2006; Raskind et al., 2007; Davis et al., 2008; Naylor et al., 2015; Petrakis et al., 2016; Villarreal et al., 2016; Ramaswamy et al., 2017; Surís et al., 2017; Raskind et al., 2018). Sample sizes for all included studies ranged from 12 to 304. A total of 16 drugs were included in this study for the handling of gainsay-related chronic PTSD, among which three studies reported risperidone and three studies reported prazosin. Guanfacine and topiramat were discussed in two studies, and the other drugs, including pregabalin, amitriptyline, fluoxetine, phenelzine, aripiprazole, vilazodone, rivastigmine, riluzole, olanzapine, dexamethasone, and quetiapine, had just one written report reporting work on PTSD. The other main features are displayed in Tabular array 1. The quality assessment is presented in Supplementary Table S1.
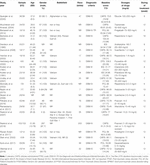
Tabular array one. Summary of included clinical trials and patient characteristics.
3.3 Results of Meta-Analysis
3.3.1 Effectiveness
3.3.1.one Changes in Total Symptoms of Combat-Related PTSD
In terms of changes in full combat-related PTSD symptoms, 7 drugs in 14 RCTs were compared with the placebo in this study. Overall active comparators [SMD = −0.36, 95%CI (−0.62, −0.09)] are shown in Figure 2 and quetiapine (SMD = −0.49, 95%CI (−0.93, −0.04)) in Table ii, which showed statistical differences. Notwithstanding, other active drugs, including topiramate, guanfacine, prazosin, vilazodone, risperidone, and olanzapine did not significantly reduce the change in total symptoms of combat-related PTSD, as outlined in Table 2.

Effigy ii. Forest for modify in total symptoms of combat-related PTSD based on a clinician-assessed scale based on CAPS scores.

Table 2. Meta-assay of different drug classifications for efficacy outcomes.
3.3.ane.two Symptom of Depression
In terms of changes in depressive symptoms, ix drugs in xiv RCTs were compared with the placebo in this written report. Every bit shown in Figure 3, overall, the active comparators [SMD = −0.28, 95%CI (−0.45, −0.ten)], both amitriptyline [SMD = −1.16, 95%CI (−i.xc, −0.41)] and quetiapine [SMD = −0.63, 95%CI (−1.08, −0.18)] significantly effected improved depressive symptoms, as shown in Table 2. However, other active drugs, including riluzole, topiramate, pregabalin, risperidone, guanfacine, prazosin, and dexamethasone had no statistically pregnant difference, every bit shown in Table 2.
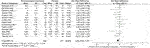
Effigy iii. Woods for symptoms of low.
3.three.1.3 Symptom of Anxiety
In terms of changes in anxiety symptoms, seven drugs in 8 RCTs were compared with placebo in this written report. Overall active comparators [SMD = −0.44, 95%CI (−0.64, −0.23)] and unlike drugs classifications in Effigy four, such as risperidone [SMD = −0.82, 95%CI (−one.46, −0.17)], amitriptyline [SMD = −0.99, 95%CI (−ane.72, −0.26)], and imipramine [SMD = −0.67, 95%CI (−one.34, −0.01)] significantly reduced the symptoms of anxiety. All the same, other active drugs, including phenelzine, quetiapine, riluzole, and pregabalin had no statistically significant departure, as shown in Tabular array 2.
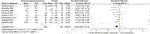
FIGURE 4. Forest for symptoms of anxiety.
3.3.ane.4 Symptoms of Re-Experiencing
In terms of changes in the symptoms of anxiety, eight drugs in ix RCTs were compared with placebo in this study. As shown in Figure v, overall, agile comparators [SMD = -−0.33, 95%CI (−0.52, −0.13)], amitriptyline [SMD = −0.75, 95%CI (−1.46, −0.04)], imipramine [SMD = −ane.07, 95%CI (−1.77, −0.38)], and quetiapine [SMD = −0.53, 95%CI (−0.98, −0.09)] had significance and effectively improved re-experiences of symptoms (Table 2). However, other active drugs, including risperidone, guanfacine, phenelzine, prazosin, and rivastigmine had no statistically significant deviation, as indicated by Table two.

Effigy 5. Woods for symptoms of re-experiencing.
iii.3.1.5 Symptoms of Abstention
Of the eight drugs in the nine RCTs compared with placebo in this study, overall the active comparators [SMD = −0.24, 95%CI (−0.43, −0.05)] shown in Figure 6 and different drug classifications, including amitriptyline [SMD = −0.xc, 95%CI (−one.62, −0.18)] and imipramine [SMD = −0.81, 95%CI (−1.49, −0.14)], significantly reduced symptoms of avoidance (run into Table two). Other agile drugs, including risperidone, guanfacine, phenelzine, prazosin, rivastigmine, and quetiapine showed no statistically significant difference (see Tabular array 2).

FIGURE six. Forest for symptoms of avoidance.
3.3.one.6 Symptoms of Hyper-Arousal
In terms of changes in the symptoms of feet, five drugs in half dozen RCTs were compared with placebo. The agile comparators [SMD = −0.26, 95%CI (−0.48, −0.03)] outlined in Figure 7, including quetiapine [SMD = −0.55, 95%CI (−1.00, −0.x)] are shown in Table 2. Other agile drugs, including risperidone, guanfacine, prazosin, and rivastigmine, had no statistically pregnant difference (see Tabular array 2).
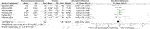
FIGURE 7. Forest for symptoms of hyper-arousal.
3.3.2 Acceptability
three.3.2.ane All-Crusade Discontinuation
The active comparators [RR = 0.97, 95%CI (0.78, 1.20)] shown in Figure 8, except for risperidone [RR = 1.77, 95%CI (1.09, 2.89)] and imipramine [RR = 0.32, 95%CI (0.12, 0.80)] did non show a statistically significant difference for all-cause discontinuance, every bit indicated past Table iii.

FIGURE 8. Forest for all-cause discontinuation charge per unit.

Tabular array 3. Meta-analysis of unlike drug classifications for acceptability.
3.three.2.2 Discontinuation due to AEs
The divergence in the overall active comparators [RR = ii.42, 95%CI (1.41, 4.xiii)] discontinued due to AEs was statistically meaning compared with placebo, come across Figure 9. As Tabular array iii reveals, of the different drug classifications discontinued due to AEs, topiramate [RR = iv.17, 95%CI (1.15, 15.xiii)] was significantly higher than placebo, simply the other agile comparators were not.

Effigy 9. Wood for discontinuation rate due to adverse events.
three.3.3 Adverse Events
Compared with placebo, overall, there was no statistically significant difference amid agile comparators in all AEs, including somnolence [RR = iv.55, 95%CI (0.25, 8.70)], sedation [RR = 5.00, 95%CI (0.26, 98.00)], dizziness [RR = ane.98, 95%CI (0.91, 4.31)], paresthesia [RR = 3.00, 95%CI (0.31, 69.52)], anxiety and blurred vision [RR = 0.33, 95%CI (0.01, seven.72)], generalized feet disorder [RR = 0.98, 95%CI (0.47, 2.04)], and slumber disturbance [RR = 1.50, 95%CI (0.28, 8.04)]) in Tabular array 4.

Table 4. Meta-analysis for the outcome of agin events.
3.3.4 Stratified Analyses
3.3.iv.i Different Ages
A pooling analysis of studies involving the consequence of drugs on gainsay-related PTSD of different ages (xviii–threescore and more than 60) showed that overall agile comparators [SMD = −0.36, 95%CI (−0.62, −0.09)− can lower the changes in combat-related PTSD symptoms, depressive [SMD = −0.28, 95%CI (−0.45, −0.x)] and anxiety [SMD = −0.44, 95%CI (−0.64, −0.23)], equally shown in Table 5. However, at that place were no patients over the age of 60 years in the RCTs.

Tabular array v. Stratified analyses for the change in total symptoms of gainsay-related PTSD, depression, and anxiety.
3.3.4.2 Dissimilar Races
Compared with placebo, overall active comparators did significantly reduce the change in total symptoms of combat-related PTSD in study participants of white [SMD = −0.49, 95%CI (−0.93, −0.04)] and other races [SMD = −0.34, 95%CI (−0.63, −0.06)]. Moreover, compared with placebo for depressive symptoms, overall active comparators had significant statistical differences in participants of white [SMD = −0.63, 95%CI (−ane.08, −0.18)] and other races [SMD = −0.21, 95%CI (−0.40, −0.03)]. Overall active comparators in white [SMD = −0.41, 95%CI (−0.86, 0.03)] participants had no significant relief of anxiety symptoms, but it had a pregnant clinical do good for anxiety [SMD = −0.44, 95%CI (−0.67, −0.22)] in participants of other races in Table 5.
3.three.4.3 Unlike Gender
Treatment of overall active comparators [SMD = −0.74, 95%CI (−1.37, −0.12)] significantly reduced the change in full symptoms of gainsay-related PTSD for male adults. Furthermore, overall active comparators [SMD = −0.18, 95%CI (−0.44, 0.07)] did not significantly relieve depressive symptoms. Yet, overall, active comparators [SMD = −0.55, 95%CI (−0.87, −0.23)] had significant therapeutic furnishings for anxiety in Table 5. Unfortunately, no RCT for female participants was involved in the systematic evaluation and meta-analysis, there was no evidence of drug treatment in female participants with gainsay-related PTSD.
three.3.4.iv Different Battleground
Based on various battlefields, the change in total symptoms of combat-related PTSD compared with placebo showed that overall, active comparators for various battlefields, including the Republic of iraq or Iran war [SMD = −ane.61, 95%CI (−2.17, −ane.06)] and unknown battlefields [SMD = −0.17, 95%CI (−0.31, −0.02)], could significantly lower the changes in full symptoms of combat-related PTSD, only for Vietnam war battlegrounds [SMD = −0.04, 95%CI (−0.58, 0.49)] there was no relief of the modify in full symptoms of combat-related PTSD. Based on diverse battlefields, low compared with placebo indicated comparative battlefields which had no significant difference for depression, including Transitional islamic state of afghanistan or Iraq [SMD = 0.03, 95%CI (−0.42, 0.49)], Republic of iraq or Iran [SMD = 0.22, 95%CI (−0.43, 0.86)], and the Vietnam war [SMD = −0.17, 95%CI (−0.56, 0.23)], just overall, active comparators for unknown battlefields [SMD = −0.32, 95%CI (−0.53, −0.10)] could relieve depression. In terms of feet, compared with placebo the overall active comparators for various battlefields, including Afghanistan or Republic of iraq [SMD = −0.16, 95%CI (−0.62, 0.30)] and Iraq-Iran [SMD = −0.26, 95%CI (−0.ninety, 0.39)], had no pregnant statistical departure, simply overall active comparators for the Vietnam war [SMD = −0.56, 95%CI (−1.01, −0.10)] and unknown battlefields [SMD = −0.55, 95%CI (−0.87, −0.22)] showed improved anxiety, see Table v.
3.3.4.5 Duration of Treatment
Compared with placebo, overall active comparators for a duration of treatment of less than eight weeks [SMD = −0.21, 95%CI (−0.50, 0.08)] did not significantly lower changes to total symptoms of combat-related PTSD. Nonetheless, overall active comparators [SMD = −0.27, 95%CI (−0.42, −0.eleven)] in the duration of drug of more than eight weeks tin can significantly lower the modify in full symptoms of combat-related PTSD. Furthermore, Compared with placebo for depressive, overall active comparators of less than 8 weeks [SMD = −0.23, 95%CI (−0.46, 0.01)] and more than than 8 weeks [SMD = −0.31, 95%CI (−0.73, 0.eleven)] had no significant statistical differences. For depressive symptoms, compared with the placebo, overall agile comparators of less than viii weeks [SMD = −0.44, 95%CI (−0.72, −0.15)] and more than 8 weeks [SMD = −0.45, 95%CI (−0.76, −0.14)] duration had significant statistical differences, every bit seen in Tabular array 5.
3.three.4.6 Severity of Trauma
Overall agile comparators in severe PTSD symptoms (60–79) [SMD = −0.20, 95%CI (−0.42, 0.01)] and extremely severe PTSD symptoms (≥80) [SMD = −0.12, 95%CI (−0.31, 0.08)] had not significantly lowered the change in total symptoms of combat-related PTSD. Moreover, compared with placebo in depression, overall active comparators in severe PTSD symptoms (60–79) [SMD = −0.13, 95%CI (−0.40, 0.xiv)] did non show significant improvement, but overall active comparators with extreme severe PTSD symptoms (≥lxxx) [SMD = −0.40, 95%CI (−0.76, −0.04)] can improve depression. Compared with placebo, overall active comparators in severe PTSD symptoms (threescore–79) [SMD = −0.27, 95%CI (−0.55, 0.01)] did not significantly improve anxiety, only overall active comparators with extreme severe PTSD symptoms (≥80) [SMD = −0.82, 95%CI (−1.46, −0.17)] can relieve anxiety, as shown in Table 5.
3.iv Publication Bias
All funnel plots were symmetrical and no publication bias was establish, as indicated in Supplemental Figures one–8.
four Word
A total of one,263 populations of combat-related PTSD participated in 22 RCTs were included in our evaluation of the clinical benefit of drugs in treating gainsay-related PTSD. Studies investigating the correlation of the different underlying factors of PTSD take found that the symptomatic characteristics of anxiety and low appeared to be more closely related to factors reflecting general anxiety than to more than specific aspects of PTSD that reflect re-experience, abstention, and hyper-arousal. This study found that guanfacine and quetiapine improved depressive symptoms positively, while risperidone, amitriptyline, and imipramine had positive furnishings on anxiety. Guanfacine is also an α2-adrenoreceptor agonist unremarkably used to treat high blood force per unit area and attention deficit hyperactivity disorder. All the same, the nightmares and sleep disturbances caused by traumatic episodes tin as well do good. Because of its long half-life, information technology was more pop than the other adrenergic drugs mentioned above. Quetiapine'south properties every bit a partial agonist of 5-HT1A and as a potent inhibitor of norepinephrine transporter through its metabolite seem to partly explicate the mechanism of major depressive disorder treatment (Cross et al., 2016). The receptor bounden properties of quetiapine were complex and it seems incommunicable to explain the effects observed in major low and anxiety disorders by a single mechanism. In addition, the amitriptyline, imipramine, and quentiapine can finer relieve the re-experiencing symptoms. The amitriptyline and quetiapine had good clinical benefits for avoidance, and the quetiapine had a skillful therapeutic effect on hyper-arousal symptoms. Information technology is important to notation that quetiapine is constructive against bipolar depression (Suttajit et al., 2014), and the present study speculates that this may exist one of the reasons why quetiapine was effective depressive symptoms. Furthermore, TCAs, including amitriptyline and imipramine, were absorbed rapidly and distributed in large quantities, and its elimination can be delayed for 10 days (Abdollahi and Mostafalou, 2014). They acted as catecholamines reuptake inhibitors, including serotonin and norepinephrine, but they besides had anticholinergic, antihistamine, and quinine effects. It is worth noting that cardiotoxicity was the main agin effect of excessive use of TCAs, and sodium bicarbonate was used to care for poisoning. This was dissimilar from other studies (Puetz et al., 2015) that looked at medications for feet and depression, in which treatment with SSRIs and TCAs accept been constitute to reduce PTSD, anxiety, and depression symptoms in veterans. SSRIs also play a role in the symptom comeback of PTSD, which was derived from the mechanism that SSRIs were neurotransmitters were released by synaptic vesicles in neurons and act on postsynaptic neurons. Neurotransmitter release was quantum release, synaptic vesicles were non released 1 by i, nor were they released in groups, they were released all at in one case and hydrolyzed rapidly. When released, it acted on the postsynaptic membrane, causing the next neuron to fire. The simplest style to do this was to reabsorb the neurotransmitters that were released, which is how neurotransmitters reabsorb. SSRI drugs acted on the reabsorption process to forbid its reuptake and continue to act on postsynaptic neurons to increment the concentration of SSRIs in the synaptic cleft, thus playing a office of anti-depression and anti-anxiety.
Squeamish Guidelines recommend the utilize of antipsychotics for people with PTSD (Excellence NIfHaC, 2019). PTSD comorbidities (McCauley et al., 2012) are treated differently for non-veterans, with higher rates of suicide attempts and poorer treatment adherence (McCauley et al., 2012; Reisman, 2016). Therefore, atypical antipsychotics may be considered more appropriate for this particular group of veterans than SSRIs. The results of the meta-analysis suggested that singular antipsychotics improved the handling of combat-related PTSD. This finding differs from a recent meta-analysis of the effects of drugs on PTSD. This study constitute that risperidone and olanzapine (Stein et al., 2002; Bartzokis et al., 2005) were suitable for veterans with extremely severe symptoms of PTSD, while there were no effective medications for veterans with severe PTSD.
In this meta-analysis, age and race were used every bit influencing factors for drug treatment of combat-related PTSD. In this study, drugs showed a skillful therapeutic upshot on veterans between 18 and 60 years one-time, but evidence on veterans over threescore years old was defective. The main cause appears to be women'south reported lower levels of armed services readiness, weaker unit cohesion, and higher incidences of depressive symptoms (Bisson et al., 2019). Unfortunately, no studies reported the treatment of female participants with drugs. In addition, the study found that anxiety tin can exist effectively reduced in veterans when they receive medication in an unknown battlefield setting, as did those with anxiety who served in Vietnam (VA/DOD, 2018). However, overall, active comparators were not effective at treating anxiety symptoms experienced by veterans of the war in Afghanistan and Iraq, or Iran and Iraq.
Considering the efficacy of different durations of treatment, long-term treatment interventions had a more significant effect on change in total symptoms of combat-related PTSD and could reduce the clinical issue of change in total symptoms of gainsay-related PTSD. All the same, the duration of intervention showed no clinical advantage in improving depression. In contrast, interventions of different elapsing did not touch the overall outcome of feet and all reduced anxiety symptoms. In addition to cumulative therapeutic efficacy, the differences in duration of treatment may also exist related to the depression sample size allotment caused past stratified analysis, which reduces the power of statistical efficacy in each subgroup. Although many factors can influence the outcome to benefit analysis, the present study recommends that clinicians keep treatment for at least 8 weeks to better manage symptoms associated with PTSD.
Imipramine was the just drug that proved to be significantly superior in terms of the rate of discontinuation for all causes (Kosten et al., 1991). Imipramine is a tricyclic antidepressant, mainly used for the treatment of major depression, obsessive-compulsive disorder, generalized feet disorder, and other psychiatric disorders, which may exist one of the reasons for the low withdrawal rate of imipramine due to AEs (Dean, 2012). After subgroup analysis, a study past Bartzokis, Hamner, and Monnelly showed a loftier upshot on the all-cause discontinuation charge per unit of risperidone (Hamner et al., 2003; Monnelly et al., 2003; Bartzokis et al., 2005). The high rate of risperidone withdrawal may be related to the dosage of risperidone. In related studies, risperidone was used in small doses with fewer side effects (Monnelly et al., 2003). In addition, the number of discontinuation due to AEs of anticonvulsive drugs increased significantly. This study also found that overall agile comparators had certain AEs, including drowsiness, sedation, dizziness, paresthesias, anxiety and blurred vision, generalized feet disorder, and sleep disturbances. Lethargy, dizziness, sedation, and other AEs that occur from the use of quetiapine, and these AEs were paid attention to by clinicians.
Based on current guidelines and evidence, this study aims to make the following recommendations for clinicians when conducting inquiry on gainsay-related PTSD. Clinicians should fully consider individual factors, including different symptoms, different populations groups, or severe sub-symptoms, when choosing drugs. Clinicians tin likewise employ the results of this study to initially identify advisable drug treatment regimens, taking into account patients' preferences (McHugh et al., 2013; Zoellner et al., 2019). For time to come research, the pharmacological handling of PTSD may need to exist studied with specific drugs that have shown strong therapeutic value in small-scale sample studies. Hereafter studies should continue to wait at this particular population of combat-related PTSD and provide a rigorous stratified analysis of baseline symptoms to identify private differences between medications.
This study has the following limitations. First of all, despite the large number of active drugs included, the sample size of included active drugs was too small, which may lead to the low statistical power of the results in this study. Therefore, the conclusions of this report demand to be verified past larger samples and high-quality RCTs in the future. Secondly, there were differences in the use of certain dosages of agile drugs, which causes heterogeneity in the results and influences the accurateness of the results to some extent. Finally, the SD of continuous outcomes was missing and unavailable, which was also one of the reasons that the number of studies in this analysis was too small and affected the results.
5 Conclusion
Active drugs, especially amitriptyline, imipramine, and quetiapine, had a positive effect and improved combat-related PTSD symptoms. Even though there was no pregnant increase in AEs of the active drugs, the fact that the discontinuation rates of these drugs, including risperidone, imipramine, and topiramate, were increased still deserves attending. Furthermore, active drugs were effective across indigenous groups and battlefields, and active drug regimens were more advisable for treating people with symptoms of farthermost astringent PTSD (≥80) or at least viii weeks former. In improver, the current evidence was only from male adults nether 60 years of age experiencing combat-related PTSD, whether this bear witness can exist extended to other populations with combat-related PTSD needs to exist confirmed past subsequent high-quality, large-sample studies.
Information Availability Statement
The original contributions presented in the study are included in the article/Supplementary Fabric, further inquiries can be directed to the corresponding authors.
Author Contributions
CZ and Q-QG conceptualized and coordinated the study. CZ developed the search strategy. J-ZY, Q-QG, J-LL, and Ten-ZL screened citations and assessed studies for eligibility. J-LL and 10-ZL extracted data. Z-XZ and R-BL performed quality assessments. J-LL and CZ performed statistical analyses. J-ZY, CZ, and Q-QG provided methodologic expertise in cognition synthesis and resolved disagreements regarding report eligibility or quality assessments. CZ and J-ZY critically reviewed the manuscript for important intellectual content. All of the authors gave final approval of the version to be published and agreed to be answerable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of whatsoever commercial or fiscal relationships that could be construed equally a potential conflict of interest.
Publisher'southward Note
All claims expressed in this article are solely those of the authors and do non necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be fabricated by its manufacturer, is non guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this commodity can be found online at: https://www.frontiersin.org/articles/ten.3389/fphar.2021.805354/total#supplementary-material
References
Abdollahi, M., and Mostafalou, Southward. (2014). Tricyclic Antidepressants in Encyclopedia of Toxicology. 3rd Edition, 838–845. doi:10.1016/b978-0-12-386454-iii.00796-ten
CrossRef Full Text | Google Scholar
Akuchekian, S., and Amanat, South. (2004). The Comparison of Topiramate and Placebo in the Treatment of Posttraumatic Stress Disorder: A Randomized, Double- Blind Study. J. Res. Med. Sci. 5, 240–244. doi:10.1186/1744-859x-5-s1-s225
CrossRef Total Text | Google Scholar
Bajor, L. A., Ticlea, A. N., and Osser, D. N. (2011). The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an Update on Posttraumatic Stress Disorder. Harv. Rev. Psychiatry nineteen, 240–258. doi:10.3109/10673229.2011.614483
PubMed Abstruse | CrossRef Total Text | Google Scholar
Baniasadi, M., Hosseini, G., Fayyazi Bordbar, 1000. R., Rezaei Ardani, A., and Mostafavi Toroghi, H. (2014). Effect of Pregabalin Augmentation in Handling of Patients with Combat-Related Chronic Posttraumatic Stress Disorder: a Randomized Controlled Trial. J. Psychiatr. Pract. 20, 419–427. doi:10.1097/01.pra.0000456590.12998.41
CrossRef Full Text | Google Scholar
Bartzokis, G., Lu, P. H., Turner, J., Mintz, J., and Saunders, C. S. (2005). Adjunctive Risperidone in the Treatment of Chronic Combat-Related Posttraumatic Stress Disorder. Biol. Psychiatry 57, 474–479. doi:10.1016/j.biopsych.2004.11.039
PubMed Abstract | CrossRef Full Text | Google Scholar
Bisson, J. I., Berliner, L., Cloitre, M., Forbes, D., Jensen, T. Grand., Lewis, C., et al. (2019). The International Social club for Traumatic Stress Studies New Guidelines for the Prevention and Treatment of Posttraumatic Stress Disorder: Methodology and Development Process. J. Trauma Stress 32, 475–483. doi:ten.1002/jts.22421
PubMed Abstract | CrossRef Full Text | Google Scholar
Cantankerous, A. J., Widzowski, D., Maciag, C., Zacco, A., Hudzik, T., Liu, J., et al. (2016). Quetiapine and its Metabolite Norquetiapine: Translation from In Vitro Pharmacology to In Vivo Efficacy in Rodent Models. Br. J. Pharmacol. 173, 155–166. doi:10.1111/bph.13346
CrossRef Full Text | Google Scholar
Davidson, J., Kudler, H., Smith, R., Mahorney, Due south. 50., Lipper, S., Hammett, E., et al. (1990). Treatment of Posttraumatic Stress Disorder with Amitriptyline and Placebo. Curvation. Gen. Psychiatry 47, 259–266. doi:x.1001/archpsyc.1990.01810150059010
PubMed Abstruse | CrossRef Full Text | Google Scholar
Davis, Fifty. L., Ward, C., Rasmusson, A., Newell, J. Yard., Frazier, Due east., and Southwick, Due south. Yard. (2008). A Placebo-Controlled Trial of Guanfacine for the Treatment of Posttraumatic Stress Disorder in Veterans. Psychopharmacol. Bull. 41, eight–18.
PubMed Abstract | Google Scholar
De Berardis, D., Fornaro, M., Valchera, A., Cavuto, M., Perna, One thousand., Di Nicola, M., et al. (2018). Eradicating Suicide at its Roots: Preclinical Bases and Clinical Evidence of the Efficacy of Ketamine in the Handling of Suicidal Behaviors. Int. J. Mol. Sci. xix, 2888. doi:10.3390/ijms19102888
CrossRef Total Text | Google Scholar
Dean, L. (2012). "Imipramine Therapy and CYP2D6 and CYP2C19 Genotype," in Medical Genetics Summaries. V. M. Pratt, S. A. Scott, M. Pirmohamed, B. Esquivel, M. S. Kane, B. L. Kattmanet al. (Bethesda (Dr.): National Centre for Biotechnology Information U.s.a..
Google Scholar
Excellence NIfHaC (2019). Guideline Postal service-traumatic Stress Disorder.
Google Scholar
Frayne, Due south. Thousand., Seaver, M. R., Loveland, Due south., Christiansen, C. L., Spiro, A., Parker, 5. A., et al. (2004). Burden of Medical Affliction in Women with Depression and Posttraumatic Stress Disorder. Arch. Intern. Med. 164, 1306–1312. doi:10.1001/archinte.164.12.1306
PubMed Abstract | CrossRef Full Text | Google Scholar
Hamner, M. B., Faldowski, R. A., Ulmer, H. Thousand., Frueh, B. C., Huber, G. Yard., and Arana, G. W. (2003). Adjunctive Risperidone Handling in post-traumatic Stress Disorder: a Preliminary Controlled Trial of Effects on Comorbid Psychotic Symptoms. Int. Clin. Psychopharmacol. eighteen, 1–eight. doi:ten.1097/00004850-200301000-00001
PubMed Abstract | CrossRef Full Text | Google Scholar
Hertzberg, Thousand. A., Feldman, M. Due east., Beckham, J. C., Kudler, H. Southward., and Davidson, J. R. (2000). Lack of Efficacy for Fluoxetine in PTSD: a Placebo Controlled Trial in Gainsay Veterans. Ann. Clin. Psychiatry 12, 101–105. doi:ten.1023/a:1009076231175
PubMed Abstract | CrossRef Full Text | Google Scholar
Julian, P. T. H., and Emerge, One thousand. (2011). Cochrane Handbook for Systematic Reviews of Interventions. United kingdom of great britain and northern ireland: The Cochrane Collaboration.
Google Scholar
Kessler, R. C., Petukhova, K., Sampson, N. A., Zaslavsky, A. M., and Wittchen, H -U. (2012). Twelve-month and Lifetime Prevalence and Lifetime Morbid Take a chance of Anxiety and Mood Disorders in the United States. Int. J. Methods Psychiatr. Res. 21, 169–184. doi:10.1002/mpr.1359
CrossRef Full Text | Google Scholar
Kessler, R. C. (2000). Posttraumatic Stress Disorder: the burden to the Private and to Social club. J. Clin. Psychiatry 61 (Suppl. 5), 4–12. discussion three-4.
Google Scholar
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., and Nelson, C. B. (1995). Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060. doi:10.1001/archpsyc.1995.03950240066012
PubMed Abstract | CrossRef Full Text | Google Scholar
Kosten, T. R., Frank, J. B., Dan, Eastward., McDougle, C. J., and Giller, E. L. (1991). Pharmacotherapy for Posttraumatic Stress Disorder Using Phenelzine or Imipramine. J. Nerv Ment. Dis. 179, 366–370. doi:10.1097/00005053-199106000-00011
CrossRef Total Text | Google Scholar
Lindley, Southward. East., Carlson, East. B., and Hill, K. (2007). A Randomized, Double-Blind, Placebo-Controlled Trial of Augmentation Topiramate for Chronic Combat-Related Posttraumatic Stress Disorder. J. Clin. Psychopharmacol. 27, 677–681. doi:ten.1097/jcp.0b013e31815a43ee
CrossRef Full Text | Google Scholar
McCauley, J. L., Killeen, T., Gros, D. F., Brady, K. T., and Back, S. E. (2012). Posttraumatic Stress Disorder and Co-occurring Substance Use Disorders: Advances in Assessment and Treatment. Clin. Psychol. (N.Y): A Publication Division Clin. Psychol. Am. Psychol. Assoc. 19 (three). doi:10.1111/cpsp.12006
PubMed Abstract | CrossRef Total Text | Google Scholar
McHugh, R. K., Whitton, S. West., Peckham, A. D., Welge, J. A., and Otto, M. Due west. (2013). Patient Preference for Psychological vs Pharmacologic Handling of Psychiatric Disorders: a Meta-Analytic Review. J. Clin. Psychiatry 74, 595–602. doi:10.4088/JCP.12r07757
CrossRef Total Text | Google Scholar
Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, 1000., Wymer, J., et al. (2018). 3,4-methylenedioxymethamphetamine (MDMA)-assisted Psychotherapy for mail-traumatic Stress Disorder in Military Veterans, Firefighters, and Police force Officers: a Randomised, Double-Blind, Dose-Response, Phase 2 Clinical Trial. Lancet Psychiatry five, 486–497. doi:10.1016/S2215-0366(xviii)30135-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Mohamed, South., and Rosenheck, R. A. (2008). Pharmacotherapy of PTSD in the U.S. Department of Veterans Affairs: Diagnostic- and Symptom-Guided Drug Selection. J. Clin. Psychiatry 69, 959–965. doi:10.4088/jcp.v69n0611
CrossRef Full Text | Google Scholar
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
PubMed Abstract | CrossRef Full Text | Google Scholar
Monnelly, E. P., Ciraulo, D. A., Knapp, C., and Keane, T. (2003). Low-dose Risperidone every bit Adjunctive Therapy for Irritable Aggression in Posttraumatic Stress Disorder. J. Clin. Psychopharmacol. 23, 193–196. doi:10.1097/00004714-200304000-00012
CrossRef Total Text | Google Scholar
Naylor, J. C., Kilts, J. D., Bradford, D. W., Strauss, J. Fifty., Capehart, B. P., Szabo, S. T., et al. (2015). A Pilot Randomized Placebo-Controlled Trial of Adjunctive Aripiprazole for Chronic PTSD in US Military Veterans Resistant to Antidepressant Treatment. Int. Clin. Psychopharmacol. xxx, 167–174. doi:10.1097/YIC.0000000000000061
PubMed Abstract | CrossRef Full Text | Google Scholar
Neylan, T. C., Lenoci, M., Samuelson, K. W., Metzler, T. J., Henn-Haase, C., Hierholzer, R. Due west., et al. (2006). No Improvement of Posttraumatic Stress Disorder Symptoms with Guanfacine Treatment. Am. J. Psychiatry 163, 2186–2188. doi:10.1176/appi.ajp.163.12.2186
CrossRef Total Text | Google Scholar
Nichter, B., Stein, M. B., Norman, S. B., Hill, K. Fifty., Straus, East., Haller, Chiliad., et al. (2021). Prevalence, Correlates, and Treatment of Suicidal Behavior in US War machine Veterans: Results from the 2019-2020 National Health and Resilience in Veterans Report. J. Clin. Psychiatry 82, 20m13714. doi:10.4088/JCP.20m13714
CrossRef Full Text | Google Scholar
Orsolini, L., Latini, R., Pompili, 1000., Serafini, Thousand., Volpe, U., Vellante, F., et al. (2020). Understanding the Complex of Suicide in Depression: from Research to Clinics. Psychiatry Investig. 17, 207–221. doi:10.30773/pi.2019.0171
PubMed Abstruse | CrossRef Total Text | Google Scholar
Petrakis, I. L., Desai, Northward., Gueorguieva, R., Arias, A., O'Brien, E., Jane, J. S., et al. (2016). Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcohol. Clin. Exp. Res. 40, 178–186. doi:10.1111/acer.12926
PubMed Abstract | CrossRef Full Text | Google Scholar
Puetz, T. Westward., Youngstedt, South. D., and Herring, Grand. P. (2015). Effects of Pharmacotherapy on Combat-Related PTSD, Anxiety, and Depression: A Systematic Review and Meta-Regression Assay. PloS one 10, e0126529. doi:10.1371/journal.pone.0126529
PubMed Abstract | CrossRef Full Text | Google Scholar
Ramaswamy, S., Driscoll, D., Reist, C., Smith, L. M., Albers, L. J., Rose, J., et al. (2017). A Double-Bullheaded, Placebo-Controlled Randomized Trial of Vilazodone in the Treatment of Posttraumatic Stress Disorder and Comorbid Low. Prim. Care Companion CNS Disord. 19, 17m02138. doi:10.4088/PCC.17m02138
PubMed Abstract | CrossRef Full Text | Google Scholar
Raskind, M. A., Peskind, Eastward. R., Chow, B., Harris, C., Davis-Karim, A., Holmes, H. A., et al. (2018). Trial of Prazosin for Mail service-Traumatic Stress Disorder in Military Veterans. Northward. Engl. J. Med. 378, 507–517. doi:10.1056/NEJMoa1507598
PubMed Abstract | CrossRef Total Text | Google Scholar
Raskind, K. A., Peskind, E. R., Hoff, D. J., Hart, 1000. L., Holmes, H. A., Warren, D., et al. (2007). A Parallel Group Placebo Controlled Study of Prazosin for Trauma Nightmares and Sleep Disturbance in Combat Veterans with mail-traumatic Stress Disorder. Biol. Psychiatry 61, 928–934. doi:10.1016/j.biopsych.2006.06.032
PubMed Abstract | CrossRef Full Text | Google Scholar
Rezaei Ardani, A., Hosseini, G., Fayyazi Bordbar, M. R., Talaei, A., and Mostafavi Toroghi, H. (2017). Consequence of Rivastigmine Augmentation in Treatment of Male Patients with Combat-Related Chronic Posttraumatic Stress Disorder: A Randomized Controlled Trial. J. Clin. Psychopharmacol. 37, 54–60. doi:10.1097/JCP.0000000000000624
CrossRef Total Text | Google Scholar
Spangler, P. T., West, J. C., Dempsey, C. L., Possemato, Thou., Bartolanzo, D., Aliaga, P., et al. (2020). Randomized Controlled Trial of Riluzole Augmentation for Posttraumatic Stress Disorder: Efficacy of a Glutamatergic Modulator for Antidepressant-Resistant Symptoms. J. Clin. Psychiatry 81, 20m13233. doi:10.4088/JCP.20m13233
PubMed Abstract | CrossRef Full Text | Google Scholar
Stein, M. B., Kline, N. A., and Matloff, J. L. (2002). Adjunctive Olanzapine for SSRI-Resistant Combat-Related PTSD: a Double-Blind, Placebo-Controlled Study. Am. J. Psychiatry 159, 1777–1779. doi:10.1176/appi.ajp.159.10.1777
CrossRef Full Text | Google Scholar
Surís, A., Holliday, R., Adinoff, B., Holder, N., and North, C. S. (2017). Facilitating Fearfulness-Based Memory Extinction with Dexamethasone: A Randomized Controlled Trial in Male person Veterans with Combat-Related PTSD. Psychiatry 80, 399–410. doi:10.1080/00332747.2017.1286892
PubMed Abstruse | CrossRef Full Text | Google Scholar
Suttajit, Southward., Srisurapanont, Grand., Maneeton, N., and Maneeton, B. (2014). Quetiapine for Acute Bipolar Depression: a Systematic Review and Meta-Analysis. Drug Des. Devel Ther. 8, 827–838. doi:x.2147/DDDT.S63779
PubMed Abstract | CrossRef Total Text | Google Scholar
VA/DOD (2018). VA/DOD Clinical Practice Guideline for the Direction of Posttraumatic Stress Disorder and Acute Stress Disorder: Clinician Summary. Focus (American Psychiatric Publishing) 16, 430–448. doi:ten.1176/appi.focus.16408
PubMed Abstract | CrossRef Full Text | Google Scholar
Villarreal, G., Hamner, Chiliad. B., Cañive, J. One thousand., Robert, S., Calais, L. A., Durklaski, V., et al. (2016). Efficacy of Quetiapine Monotherapy in Posttraumatic Stress Disorder: A Randomized, Placebo-Controlled Trial. Am. J. Psychiatry 173, 1205–1212. doi:10.1176/appi.ajp.2016.15070967
PubMed Abstract | CrossRef Full Text | Google Scholar
Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jönsson, B., et al. (2011). The Size and burden of Mental Disorders and Other Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 21, 655–679. doi:x.1016/j.euroneuro.2011.07.018
PubMed Abstract | CrossRef Full Text | Google Scholar
Zoellner, 50. A., Roy-Byrne, P. P., Mavissakalian, M., and Feeny, N. C. (2019). Doubly Randomized Preference Trial of Prolonged Exposure versus Sertraline for Handling of PTSD. Am. J. Psychiatry 176, 287–296. doi:10.1176/appi.ajp.2018.17090995
PubMed Abstract | CrossRef Total Text | Google Scholar
truesdalegeon1937.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fphar.2021.805354/full
0 Response to "Treating Combat Related Ptsd and Combat Operational Stress Reaction a Clinicians Review"
Post a Comment